Also being of an increases size tends to increase the nucleopihlicity of an atom. Click again to see term.
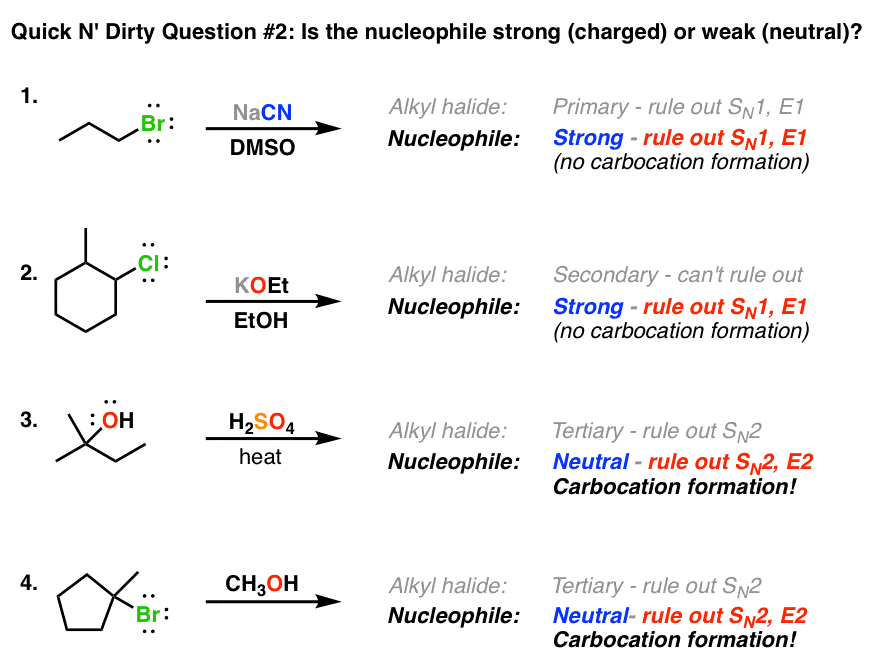
Deciding Sn1 Sn2 E1 E2 2 The Nucleophile Base
Factors affecting S N 2 mechanism.

. The rate-determining step of this reaction depends on the interaction between the two species namely the nucleophile and the organic compound. Neutraluncharged nucleophile Small neutraluncharged nucleophile Small negatively charged nucleophile Bulky nucleopha Question 23 2 points A carbon with a positive charge often seen as a reactive intermediate in organic chemistry is known as carbanion carbocation free radical none of the above. Nucleophilicity Because the nucleophile is involved in the rate-determining step of SN2 reactions stronger nucleophiles react faster.
This is VERY important throughout organic chemistry but will be especially important when trying to determine the products of elimination and substitution E1 E2 SN1 SN2reactions. For example the dissociation of NaCl in water will produce a nucleophile Cl. For an SN2 reaction - which makes the best nucleophile.
What are strong nucleophiles. Concentration of the nucleophile does not affect the rate of an SN1. - Small negatively charged nucleophile -Bulky nucleophile -Small neutraluncharted nucleophile -Neutraluncharged nucleophile The observations in elimination reactions can generally predict the products by using Zaitsevs rule which is.
Sn2 reaction is ___ molecular. The S N 2 reaction mechanism involves the nucleophilic substitution reaction of the leaving group which generally consists of halide groups or other electron-withdrawing groups with a nucleophile in a given organic compound. The SN1 Tends To Proceed In Polar Protic Solvents.
It is assumed that the nucleophile attacks the carbon atom attached to the halogen atom from the side opposite to the halogen ie. A nucleophile donates an e pair to the substrate carbon usually an organic halide or tosylate and displacing a leaving group. Nucleophile strength Carbon skeleton Leaving group Solvent.
The S N 2 reaction is a type of reaction mechanism that is common in organic chemistryIn this mechanism one bond is broken and one bond is formed synchronously ie in one step. The rate of S N 2 reaction is increased when strong nucleophiles are used. For an SN2 reaction - which makes the best nucleophile.
The S N 2 reaction is favored by polar aprotic solvents these are solvents such as acetone DMSO acetonitrile or DMF that are polar enough to dissolve the substrate and nucleophile but do not. You would actually need a really strong nucelophile bases can be that but strong nucelophiles can also not be bases. Protic polar solvents will reduce the reactivity of this nucleophile.
The use of protic solvents those such as water or alcohols with hydrogen-bond donating capability decreases the power of the nucleophile because of strong hydrogen-bond interactions between solvent protons and the reactive lone pairs on the nucleophile. Which species are present in the transition state of the rate-limiting step. Click to see full answer.
An example of an SN2. -The more substituted double bond will yield the main. Polar aprotic solvents are best suited for S N 2 reactions as polar solvents create nucleophiles.
In the above reaction of S N 2 example OH acts as a nucleophile attacks and simultaneously chlorine which is a good leaving group leaves OH comes and attaches at that point. Herein what is a strong nucleophile for sn2. A reaction where an electron-pair donation by a nucleophile to an atom usually carbon displaces a leaving group from the same atom in a concerted manner.
2 The reactivity of the nucleophile. PPh3 is a good enough nucleophile to do SN2 reactions of primary and secondary alkyl halides. Tap again to see term.
Thats why we use aprotic solvents in the case of SN 2 reactions because the concentration of the nucleophile affects the rate of the reaction even though we have to comprise a little bit on the idea of stabilisation charge on any element throughout the course of the reaction. Since two reacting species are involved in the slow rate. S N 2 is a kind of nucleophilic substitution reaction mechanism the name referring to the Hughes-Ingold symbol of the mechanism.
Can occur concurrently with SN1 reactions since both occur under similar conditions type of halide carbocation intermediate neutral nucleophilebase Examples. The mechanism of SN 2 reaction involves a single step. An SN2 reaction is a nucleophilic substitution reaction where the leaving nucleophile disassociates and the attacking nucleophile binds to the molecule simultaneously.
In fact there is not a more important part of an organic chemistry reaction than the nucleophile and the electrophile. The rate of an SN2 reaction is significantly influenced by the solvent in which the reaction takes place. Therefore the breaking of carbon halogen C X bond and making of carbon nucleophile C OH bond occurs simultaneously.
H2O ROH H2S RSH Strong Non-Nucleophilic Bases SNNB Usually anions that are very sterically hindered preventing them from attacking as nucleophiles thus the. The reaction is ______. Strong nucleophiles tend to be negatively charged and good bases.
For SN2 the rate determining step is the steric hindrance.

Solved For An Sn2 Reaction Which Makes The Best Chegg Com
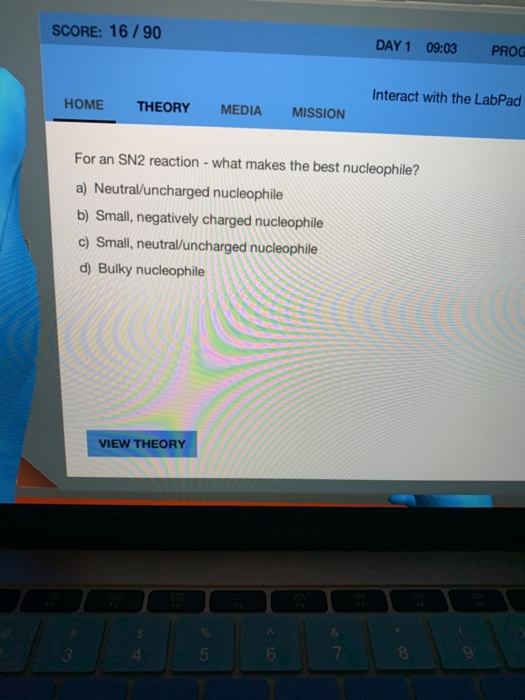
Solved Score 16 90 Day 1 09 03 Prog Interact With The Chegg Com
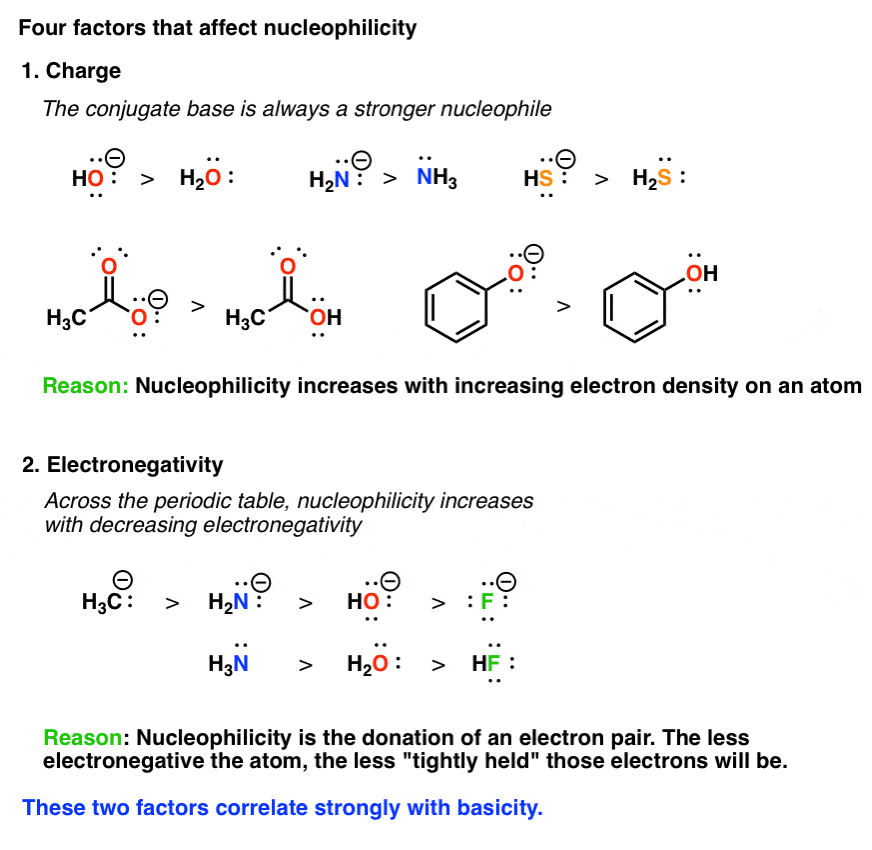
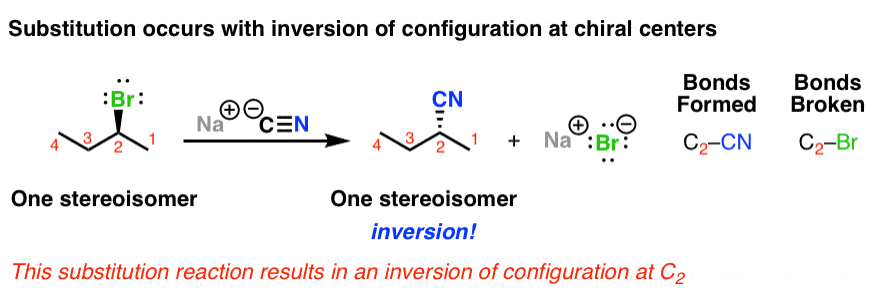
0 Comments